Describe How Acidic Solutions Differ From Pure Water
A concentrated strong base has a high pH valuethe pH of concen-. Describe how acidic solutions differ from pure water.

The Acid Base Properties Of Water Video Lesson Transcript Study Com
That means its neither an acid or a base.

. As temperature increases the pH of water decreases. All acidic solutions contain more hydrogen ions than hydroxide ions. This is due to gaseous substances dissolved in water.
Acetic acid in the buffer solution will react with the addition of. Acid and Bases I. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base.
Acidic solutions have a lower pH while basic solutions have a higher one. The OH-ion gives basic solutions their characteristic properties. Household items with a pH above 7 common examples include baking soda and soap are alkaline.
The pH of rain water is around 56. Acidic solutions are made by dissolving the acidic compound as the solute in water as the solvent. The pH of water is a very important measurement concerning water quality.
Water is not a buffer solution and the acetic acidacetate solution is a buffer solution. How do acidic solutions differ from water. H 2O H aq OH-aq.
Acid water is classified as water with a pH value lower than 7. There is an equilibrium between these two ions in water or in any aqueous solution. PH is a measure of how acidicbasic water is.
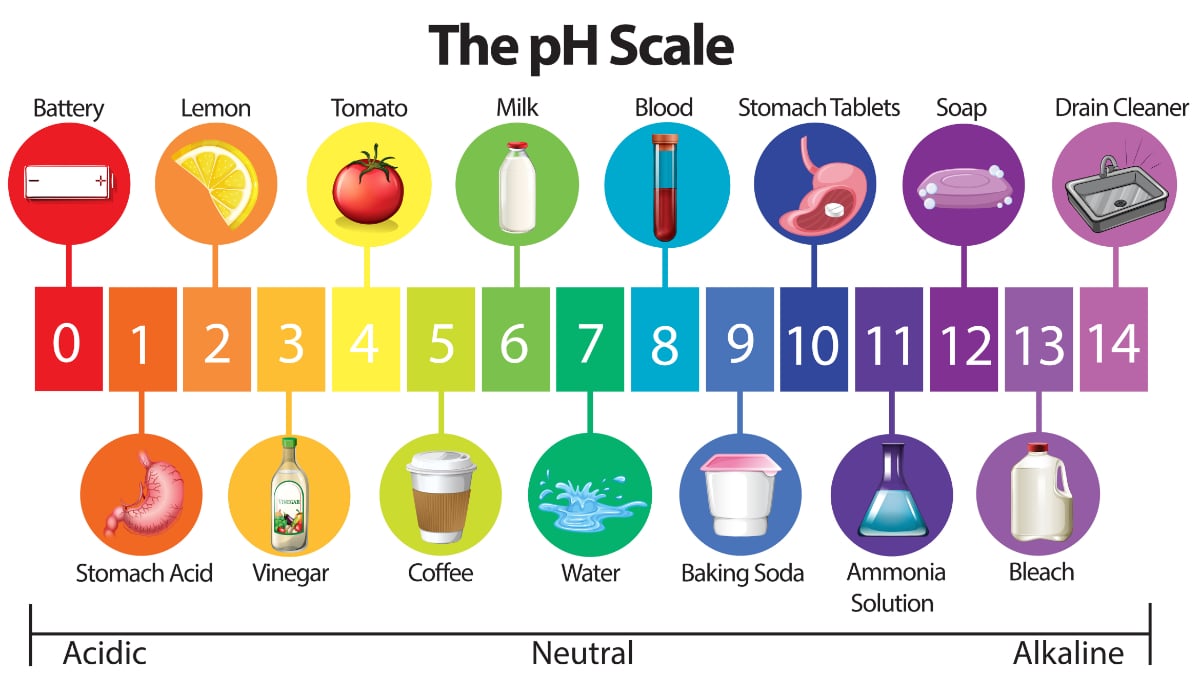
Strong acids dissociate completely in aqueous solution and have negative values for Ka. Pure water has a pH of 7 while acidic solutions will ALWAYS have a pH lower that 7. Pure water has a pH of 7.
Household liquids considered naturally acidic are coffee and vinegar. A neutral substance such as pure distilled water has a pH of 7. A solution that has a pH of 10 is twice as acidic as a solution that has a pH of 20.
In pure water the concentration of solvated protons equals the concentration of solvated hydroxide anions and the pH is 7. A n ___ is any substance with a ph under 70. A concentrated strong acid has a low pH valuethe pH of concentrated hydrochloric acid for example is less than 0.
Acidic solutions contain higher concentrations of H ions than pure water and have pH values below 7. A substance is neither an acid nor a base. A basic solution contains lower concentration of H ions than pure water and pH values above 7.
Acidic and Basic water solutions. On the pH scale acidic solutions are below 7 and are increasingly acidic and pure water is at 7 making it neutral. When fossil fuels are burned and their products interact with water in the atmosphere acid precipitation can occur.
Dissociation of water The H ion or the H 3O ion is characteristic of acidic water solutions. The pH of acidic solutions is less than 7. The pH of pure water is 7 at 25 o C.
In water and neutral solutions the concentration of hydrogen ions is equal to the concentration of hydroxide ions. An acidic buffer is a solution of a weak acid acetic acid and its conjugate base pair sodium acetate that prevents the pH of a solution from changing drastically through the action of each component with incoming acid or base. Any solution with a pH under 7 is an acid and any solution with a pH over 7 is a base.
Acidic solutions contain higher concentrations of H ions than pure water and have pH values below 7. Numbers above 7 indicate a basic solution. The range goes from 0 to 14 with 7 being neutral.
How do acidic solutions differ from water. Numbers below 7 indicate acidic solutions.
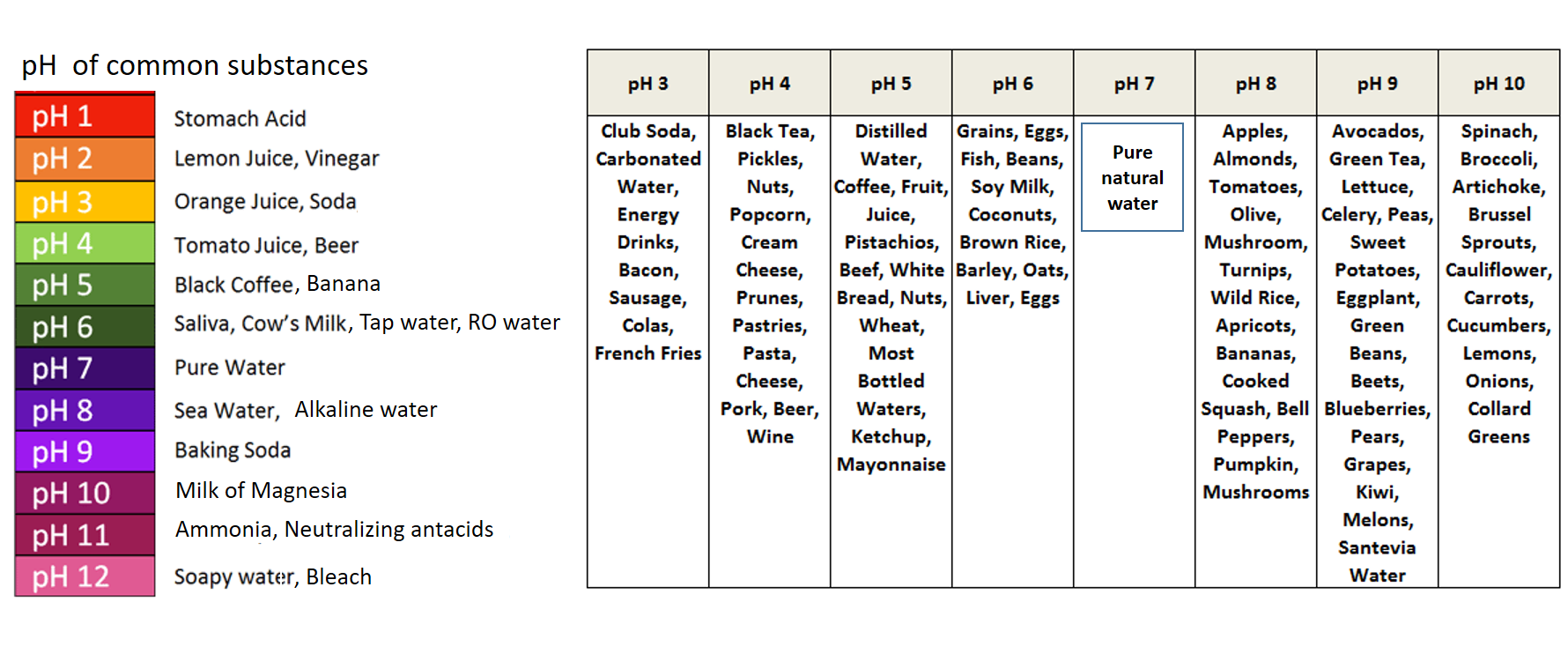
Drinking Water Can Be Acidic Or Alkaline Based On Salts And Source

No comments for "Describe How Acidic Solutions Differ From Pure Water"
Post a Comment